how to draw molecular orbital diagram of n2
Also calculate its bond order and predictits magnetic behaviour. Determine the electron configuration and bond order for each and rank the three species in order of increasing bond order 1.

N2 Contains A Triple Bond What Molecular Orbitals In N2 Contribute To The Triple Bond Do You Think Each Of The Molecular Orbitals Involved In The Triple Bond Contributes Equally To The
The leftover two 2p orbitals.
. Answered 4 years ago. Hard View solution Use the molecular orbital energy level diagram to show that N 2 would be expected to have a triple bond F 2 a single bond and Ne 2 no bond. So the bond order of nitrogen is 3.
The leftover two 2p orbitals become two π bonds and electrons making a pair between the nitrogen atoms will make a sigma bond. The bond order of N2- is also calculated and the meaning of this nu. The other is for AFTER nitrogen start.
A Number of electrons in bonding molecular orbitals. Molecular orbital diagram of N 2 BO Nb-Na 10-4 3 Since all the electrons in nitrogen are paired it is diamagnetic molecule. - N 2 molecules are diamagnetic with no unpaired electrons.
From the diagram we can see. σ 1 s 2 σ 1 s 2 σ 2 s 2 σ 2 s 2 Π 2 p x 2 Π 2 p y 2 σ 2 p z 2. One atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons.
Figure A partial molecular orbital energy-level diagram for the HF molecule. Hard View solution View more Get the Free Answr app. Next 2 in 1s sigma anti bond orbital.
Bond order 3. This video discusses how to draw the molecular orbital MO diagram for the N2- molecule. Bond order 1 2 Number of electrons in BMO Number of electrons in ABMO Bond order 1 2 8 2 Bond order 1 2 6 Bond order 3.
In the molecular orbital diagram for the molecular ion N 2 the number of electrons in the σ 2p molecular orbital is. Electronic configuration of N 2 14 electrons σ1s 2 σ1s 2 σ2s 2 σ2s 2 π 4 2p z 2. Bond order formula is given as below.
σ 1 s 2 σ 1 s 2 σ 2 s 2 σ 2 s 2 Π 2 p x 2 Π 2 p y 2 σ 2 p z 2. With the help of molecular orbital theory draw the molecular orbital energy level diagram for N_ 2 ion molecule. For N X 2 the orbitals in.
Next 2 in 2s sigma bond orbital. There are two MO diagrams you need to memorize for diatoms N2 O2 Ne2 etcOne is for the elements up to Nitrogen. The diagram above is the molecularN2 molecular orbital energy level diagram picture is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web the molecular orbital.
Number of electrons in antibonding orbitals. Molecular orbital diagram of no. Electronic configuration of N 2 is.
Hence the electronic configuration of N 2 ion will be. Molecular orbital diagram for nitrogen gas N2Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s2sigma2s2pi2p4sigma2p2Bond Or. From the diagram we can see that in N 2 all the electrons are paired and hence the magnetic moment of this compound is zero.
So the bond order of nitrogen is 3. The bond order is Figure The molecular orbital energy-level diagram for both the NO and CN-ions. When two Nitrogen atoms bond the sigma2p bonding molecular orbitals are lower in energy than the pi2p bonding orbitalsN2- has a bond order.
So the formula to find bond order is. σ 1 s 2 σ 1 s 2 σ 2 s 2 σ 2 s 2 Π 2 p x 2 Π 2 p y 2 σ 2 p z 2. Let me explain the molecular orbital diagram of N2 using its diagram.
Draw the mo diagram for n2. B Number of electrons in antibonding molecular orbitals. VSEPR model assumes that molecular geometry minimizes the.
I Structure of N 2. Next 2 in 2s sigma anti bond orbital. N_ 2 अण क आरख चतर दवर.
This interaction introduces an element of s-p mixing or hybridization into the molecular orbital theory. There are two MO diagrams you need to memorize for diatoms N2 O2 Ne2 etcOne is for the elements up to Nitrogen. In the Lewis structure of the N2 molecule there is a formation of a triple covalent bond represented by three lines between two atoms of Nitrogen.
आणवक ऊरज सतर क आधर पर. This is because according to molecular orbital theory it has fewer electrons in bonding orbitals. So first 2 electrons go in 1s sigma bond.
Even rather simple molecular orbital MO theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed from N2 O2 F2 Ne2 the complexity of the molecular orbitals develop in two waysDraw the molecular orbital diagram for Ne 2 and determine if the bond between the two atoms will be stable. We assume that orbital order is the same as that for N2.

Mo Diagram Of Nitrogen Molecule And Its Ions Youtube
How Is The Molecular Orbital Diagram Of N2 Determined Quora
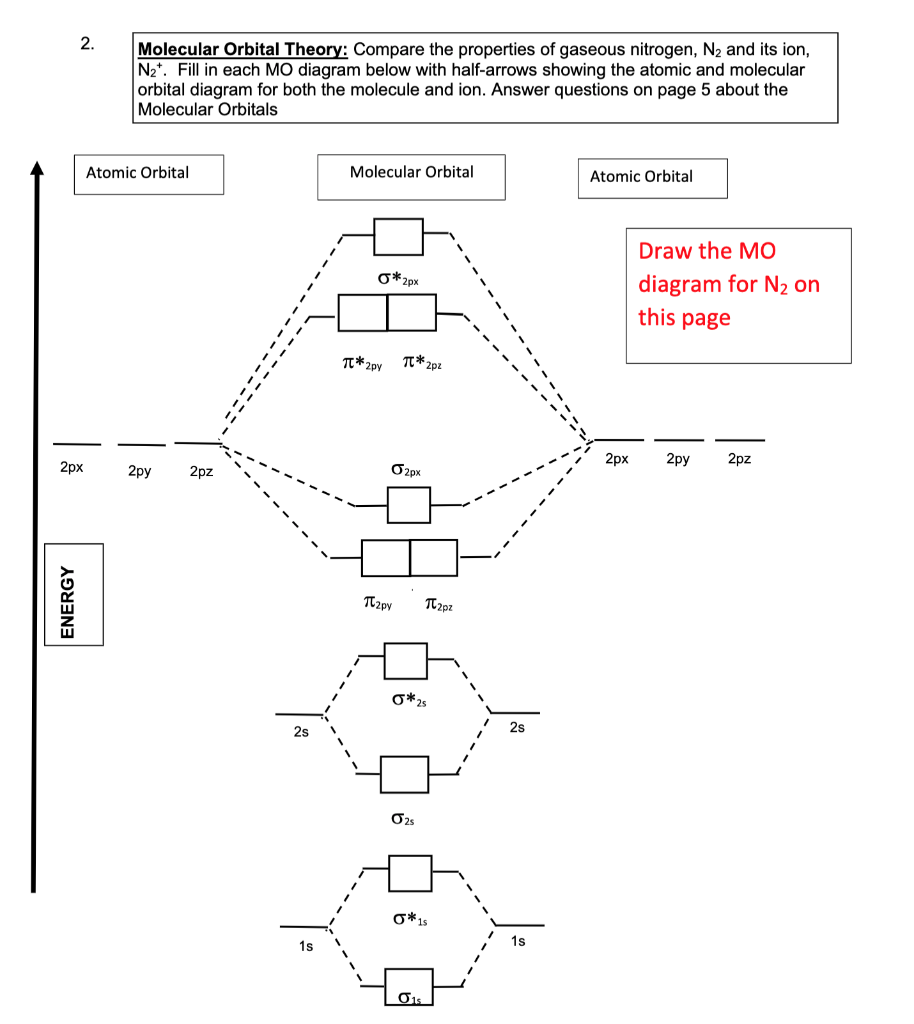
Solved Molecular Orbital Theory Compare The Properties Of Chegg Com

Molecular Orbital Diagram Of B2 C2 And N2 Molecules Youtube

Draw The Molecular Orbital Energy Level Diagram Of N2 Molecules
Draw The Molecular Orbital Diagram Of N2 Also Find Its Bond Order And Magnetic Character Chemistry Topperlearning Com 4s4p942zz

How Does The Lewis Structure Of N2 Related To The Molecular Orbital Bonding Model Quora
How Is The Molecular Orbital Diagram Of N2 Determined Quora
How Is The Molecular Orbital Diagram Of N2 Determined Quora

How To Draw The Molecular Orbital Diagram Of N2 Bond Order Of Nitrogen Molecule Chemistry Youtube

Molecular Orbital Energy Level Diagram Of Nitrogen Oxygen Youtube

Draw The Molecular Orbital Diagram Of N2n2 N2 Write Class 11 Chemistry Cbse
Draw The Diagrams For No 2 No 2 And No 2 The Homo Of No 2 Shows It Is Somewhat Anti Bonding Would You Expect The Nonbonding Electron Pairs On Nitrogen Or Oxygen To Be More Reactive

8 4 Molecular Orbital Theory Chemistry

Molecular Orbital Mo Diagram For N2 Youtube

Molecular Orbital Mo Diagram Of N2 Youtube

Draw A Molecular Orbital Diagram Of N2 Or O2 With Magnetic Class 11 Chemistry Cbse
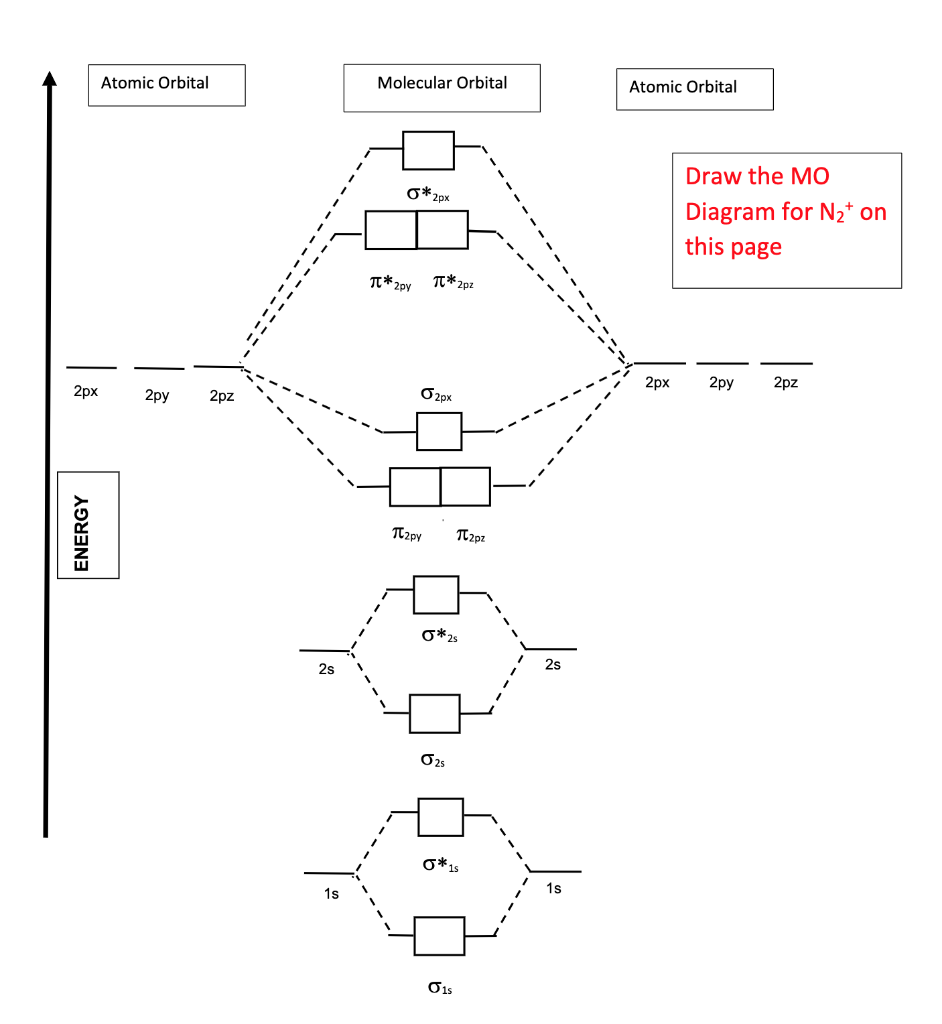
Solved Molecular Orbital Theory Compare The Properties Of Chegg Com
